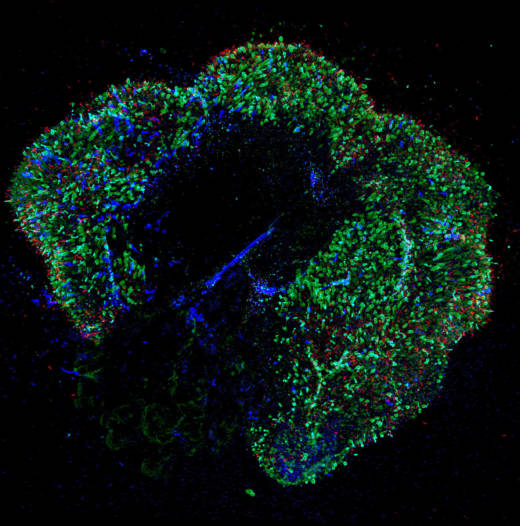
These "retinal organoids" have been around for a few years but are difficult and tedious to grow, says Kiara Eldred, a graduate student at Hopkins who is the paper's first author.
It takes up to a year to turn a batch of immature retinal cells into a functioning organoid.
"For the first week of their life, I take care of them every day," Eldred says. After a couple of weeks, she says, the cells become "a little bit more independent."
And with luck, these clusters of immature cells develop into a 3D structure that "looks and acts like a developing retina that you would see in a baby," says Bob Johnston, Eldred's boss and an assistant professor in the biology department.
Johnston's lab had been studying vision in flies. But he and Eldred saw a chance to try something much more ambitious.
"We discussed this crazy idea of: Could we use these human retinal organoids to study how we get the different color-sensing cells in our eyes?" Johnston says.
Using human cells was key because you can't study how humans see color in a fly, or even a mouse.
"Mice don't sense red," Johnston says. "They don't have these red-detecting cells. So we really have to study this in human tissue to get any insight into how it works."
Johnston and Eldred knew that in a human fetus, cells that detect blue light appear first. Then come cells that respond to red and green light.
And research on animals suggested that the thyroid hormone was involved in the development of these color-sensing cells, called cones. So Johnston had Eldred conduct an experiment with immature retinal cells.
"I added thyroid hormone to the dish during their development, and we got more red-green cones developing in those organoids," she says. "We got really excited because we were on to something."
It would take years and many more experiments to confirm that the thyroid hormone was actually triggering the emergence of color vision. And the team still hasn't figured out what causes some cones to go on to become even more specialized by detecting only red or only green.
But Johnston says his lab is now preparing to take on two new goals.
"One is to restore color vision to people that are colorblind," he says. What his lab is learning, he says, could help speed an existing effort to use gene therapy to cure colorblindness.
The lab's second goal is to use retinal organoids to better understand diseases including glaucoma and macular degeneration, a leading cause of vision loss.
Macular degeneration affects the macula, an area of the retina that provides high-resolution vision. The condition has been hard to study, though, because mice don't have a macula.
So Johnston hopes to learn more by having his lab create macular organoids.
There's growing optimism among scientists that new treatments for retinal diseases will emerge from such efforts, Becker says. Initially, he says, researchers had doubts about whether a retina grown in a dish could mimic the real thing.
But studies like this one on color vision, he says, "show that the similarity is quite high."
To encourage scientists to develop more retinal organoids, the National Eye Institute is sponsoring a scientific competition with $1 million in prizes.
Copyright 2018 NPR. To see more, visit http://www.npr.org/.
9(MDAxOTAwOTE4MDEyMTkxMDAzNjczZDljZA004))
9(MDAxOTAwOTE4MDEyMTkxMDAzNjczZDljZA004))