CRISPR: What You Need to Know About the Medical Science 'Breakthrough of the Year'
CRISPR: What You Need to Know About the Medical Science 'Breakthrough of the Year'
Editor's Note: One of our most fascinating stories of 2015 was this one, about a dramatic breakthrough that could help children suffering from the tragic illness known as "Bubble-Boy Disease." This breakthrough in gene editing, using a tool called CRISPR, is revolutionizing medical research. Science magazine has named CRISPR the 2015 Breakthrough of the Year. We present this story again here, in case you missed it in March.
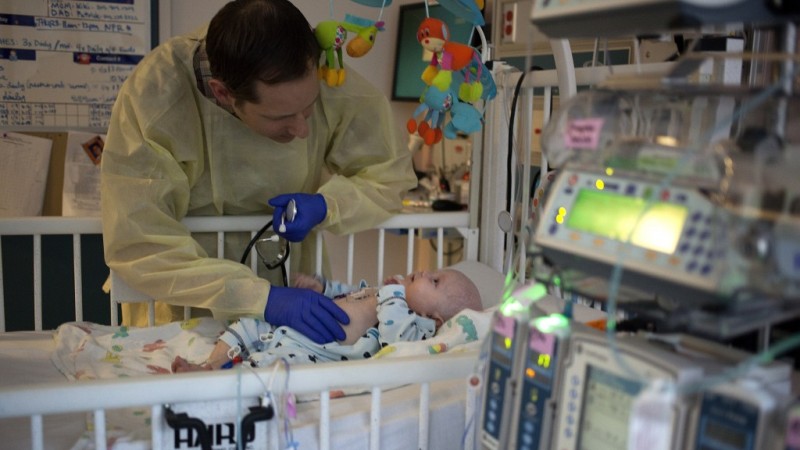
They named him Phoenix because he was born five weeks early while his parents were on vacation, and spent his first few weeks in an incubator. Kristen and Patrick Wilkinson thought they knew exactly which ashes their son might soon rise from.
But when they got him home to San Francisco things just got worse, Kristen says.
Phoenix wasn’t gaining weight. He had a persistent skin rash. Eventually he was admitted to UCSF Benioff Children's Hospital with a diagnosis of “failure to thrive.”
Phoenix had been born in Kentucky, a state where, unlike in California, infants are not routinely screened for a disease called SCID -- Severe Combined Immunodeficiency. So at first, California doctors puzzled over what might be wrong with him.
But Dr. Jennifer Puck, a SCID specialist at UCSF who pioneered the screening test, says Phoenix’s rash was the hallmark of an almost completely deficient immune system.
(The Jeffrey Modell Foundation maintains a map of states that have implemented infant SCID screening (PDF), as well as a list of states.)
“The baby’s rogue [immune] cells were attacking his own skin,” says Puck.
Left unchecked, she says, SCID is fatal. “Babies who don’t have this immunity start out getting one infection after another and can’t get over any of them.”
From Bubbles to Transplants
A generation ago, SCID was commonly known as “bubble boy disease,” named after the kids (including one portrayed by John Travolta in a 1976 made-for-TV movie) who spent their lives in plastic bubbles to shield them from germs.
Nowadays SCID babies receive bone marrow transplants. Healthy donors contribute bone marrow capable of producing infection-fighting white blood cells.
Transplanted into the blood stream of a SCID baby, the donated cells take up residence in the infant’s bone marrow and begin building a functional immune system.
But transplants are tough on babies and their families.
It can be difficult to find a good donor match. And even once one is found, most SCID babies must undergo chemotherapy to prepare their bodies for the transplanted cells.

Kristen Wilkinson says the chemo was the hardest part.
Phoenix had painful sores on the inside of his mouth. He stopped breastfeeding, had to be fed intravenously.
“He was throwing up, really nauseous,” she says. “Anytime he was awake he was screaming. He never was awake and not screaming.”
Hope for Future Treatment
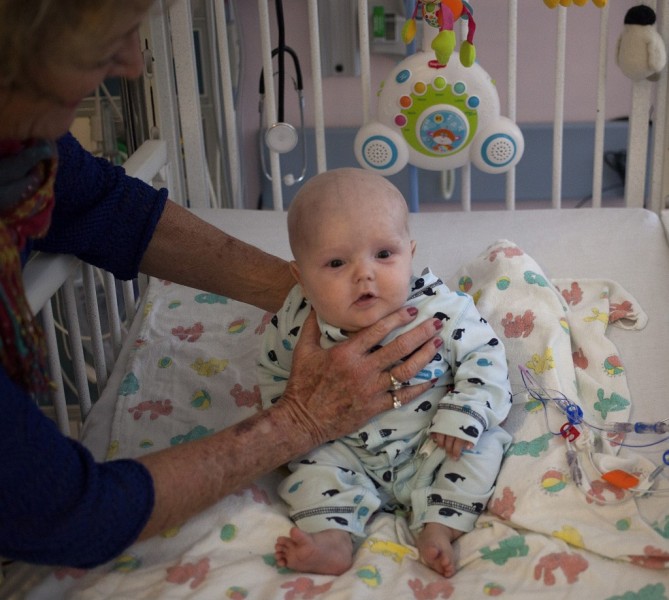
Today Phoenix is six months old, home with his parents and doing well. But Dr. Puck and others want better treatment for babies like him.
The best idea, says Puck, would be to fix the SCID mutation in Phoenix’s own cells: Take them out, reprogram them so that they can make a functional immune system, and then put them back into his body.
Phoenix would be his own donor. There would be no search for a donor match, no fear of rejection, no chemo.
“Actually just correcting the spelling mistake of the gene in the person, and not putting in any new genetic material is very attractive,” Puck says. "That should be safer.”
Gene therapy, as this is called, is not a new strategy. But it’s proved to be a challenging one.
One way to explain why is to picture a string of Christmas tree lights containing one broken bulb.
A Promising Treatment Backfires
That string is the DNA, wound up inside the nucleus of a cell. The broken bulb is the mutation.
Say you want to replace the broken bulb, but the string is too small to see. How do you know you’re taking out the right bulb and putting a new one in the correct place?
In the 1990s, doctors in Europe tried this kind of fix on a group of babies with SCID, with mixed results.
“In placing the correct copy of the gene into a chromosome, we couldn’t direct where it was going to land,” says Puck. “And sometimes it landed in a dangerous spot.”
The “dangerous spot” in this case, was next to a growth factor gene that was inadvertently turned on by the insertion.
“Growth factor genes incorrectly turned on are called oncogenes,” explains Puck. “Cancer genes.”
Five children got Leukemia, induced by a treatment that had been intended to save their lives.
Four of them recovered. One baby died. Puck says it was a devastating time for the entire field.
“The whole world mourned the loss of that baby,” she says.
Enter CRISPR
Today, Puck is excited about a new tool for making precise changes in a baby’s genome. It’s called CRISPR, an acronym for “clustered regularly interspaced short palindromic repeats.”
As UC Berkeley biologist Jennifer Doudna puts it, think of CRISPR as “a molecular scalpel.”
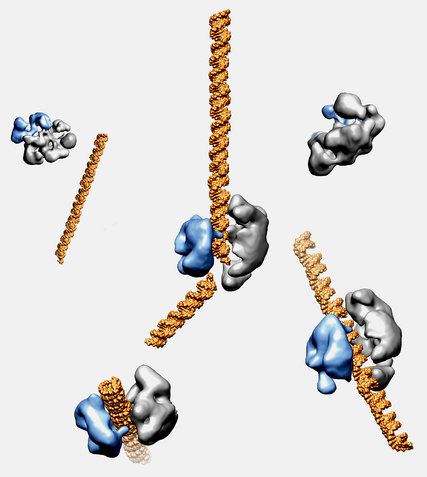
Doudna isn’t a medical doctor, or even a medical researcher. She studies bacteria -- more specifically, an enzyme, called Cas9, produced by bacteria.
Previous scientists had noticed that these bacteria possess an unusually effective way of fighting off viruses. The bacteria were able to recognize viral DNA and use Cas9 to precisely disable the virus.
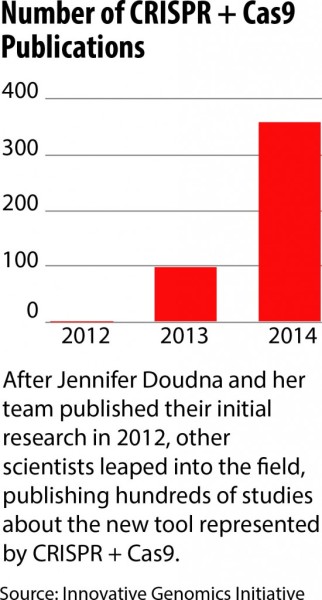
In 2012, Doudna and collaborators, including Emmanuelle Charpentier, took this a step further in a paper in the journal Science that thrilled cell biologists around the world.
The paper showed that biologists could program Cas9 -- arm it, in other words -- with a customized DNA payload.
They could direct the enzyme at virtually any cell, programmed to make whatever genetic change they wanted.
In other words, says Doudna, “scientists can go into the DNA and make a precise change that corrects the mutations that give rise to disease."
A “State of Wonder”
Fixing the DNA in a cell: that idea had been around for a while. But CRISPR works dramatically better than the old ways. It’s more accurate, faster. A process that could take years of trial and error now took an inexpensive few weeks.
“Many scientists are just -- they're sort of in a state of wonder about this,” Doudna says.
On the day we met, Doudna was showing visitors around a brand-new research center at UC Berkeley, the Innovative Genomics Initiative, which is devoted to CRISPR research.
There are now several such centers around the world and hundreds of researchers working with CRISPR on the cells of mice, cows, monkeys, plants and humans.
“CRISPR works in almost every organism it’s been tried in,” says Harvard geneticist George Church. “Dozens.”
Church is quick to point out that there are currently no CRISPR-based treatments in clinical trials. The technology is too new.
He believes this could change in a few years, though for which disease, it’s hard to say.
Church reels off a few of what he thinks might be early contenders: “Sickle cell anemia, clotting diseases, muscular dystrophies, blindness.”
“Oodles and Oodles of Money”
If CRISPR is a big deal for people who have these diseases, it’ll also be a big financial deal for the scientists who helped develop it.
And by big, Jacob Sherkow, an associate professor at New York Law School and author of a recent paper on CRISPR in Nature Biotechnology, “big” means “oodles and oodles of money. Potentially in the $200 billion to $300 billion range.”
 This windfall, he says, could go to whoever holds key patents on CRISPR.
This windfall, he says, could go to whoever holds key patents on CRISPR.
As has been widely reported, even though the University of California filed Doudna and Charpentier's application first, a key CRISPR patent went to another researcher -- Feng Zhang at MIT’s Broad Institute, which filed afterwards, but paid an additional fee to have its application expedited.
On the subject of CRISPR patents, Doudna says only that she is “very confident in the UC position.”
If Zhang’s patent prevails, Doudna and her collaborators could find themselves in the position of having to license a technology they are widely credited with discovering.
“We’re very confident that we’re going to be able to do incredible science," she says, "and we’ll let the dispute play out as it will.”
Sherkow says there are a number of ways this dispute could play out.
The US Patent Office could deny UC’s application and let Zhang's patent stand. Or it could reverse its earlier decision, revoking the Broad patent and awarding it to Doudna and her collaborators instead.
Doudna and Zhang both point to a third possibility: that among what will likely be dozens of CRISPR patents it could be difficult, at this point, to know which one will be most lucrative or important.
These early CRISPR patents, says Zhang “are just the first ones. There will be many more.”
There’s also a fourth possibility: that CRISPR could disappoint everyone, a “tempest in the teapot,” as George Church puts it.
"But I hope that’s not the case,” he says, laughing.
