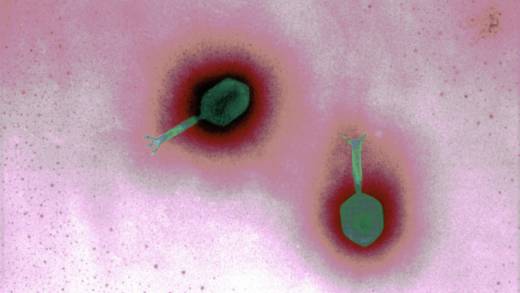
Getting phage therapy to a patient can be a bit a puzzle. These viruses are picky about the microbes they feast on, so you often need to take a swab of the patient’s bacteria, nurture it in a dish, and then test which phages are able to kill it off. You need to make sure that the phages in question will explode a bacterial cell, rather than settling comfortably in its nucleus like lice on a kindergartener’s scalp. And then you need to purify it before delivery, so there aren’t any bacterial leftovers that might poison the person instead of saving them.
There’s also plenty of bureaucracy, because phages have not been approved by the Food and Drug Administration.
It’s often a crazy rush to find the right phage with emails and calls and tweets, then getting emergency experimental approval — and that was largely what happened for the five other patients who’ve been treated with phages at UCSD since Strathdee’s husband was revived.
“We wanted to make it so it isn’t such a scramble,” said Dr. Robert “Chip” Schooley, an infectious disease specialist at UCSD, who administered the phage to Strathdee’s husband, and who is also co-director of the new center.
The announcement is also symbolic of a wider shift. With the rise of antibiotics in the 1930s and ’40s, phages went out of fashion in the U.S. But the person who had named them, a Canadian microbiologist named Felix d’Herelle, moved to Tbilisi, in the republic of Georgia, continuing his research at an institute that attracted the admiration of Joseph Stalin himself. Even after d’Herelle’s death, the Eliava Institute kept the flame of phage therapy alive.
That hardly helped the viruses’ reputation in America during the Cold War. “It was commie science; there was a taint to it,” explained Dr. William Summers, a phage biologist, historian, and professor emeritus at Yale University.
Even though phages continued to be an important part of lab science, the researchers who used them thought they were good for just that: research. The idea of using them for therapy was almost a joke.
Then, as antibiotic resistance grew into a worldwide crisis — one that kills some 23,000 Americans a year — that joke started sounding more and more appealing. The funding of a center to administer and collect data on phage therapy is a reversal, of sorts: An admission that this long-disparaged idea is worth a million-dollar second glance.
“Trust me, at the Eliava, we have tried to convince people that phages are a safe and good alternative to antibiotics for many years,” said Mzia Kutateladze, the director of the Eliava Institute. “Finally the people agreed to use it, and we are very happy, of course.” She estimated that Eliava’s phage therapy center gets around 15 to 20 Americans every year.
“There really is, thankfully, some momentum building … around these non-traditional therapies,” said Dr. Helen Boucher, an infectious disease specialist at Tufts Medical Center in Boston, who is not involved with the UCSD project. “I would think of this more in a high-risk, high-reward category. This is largely uncharted territory. … At the end of the day, if you have a product that can work against antibiotic-resistant organisms that isn’t antibiotics, that would be huge.”
The news that her brainchild had been funded took Strathdee by surprise in late May. She was at a ceremony for UCSD professors with endowed chairs, at which all of them received medals. “The chancellor’s literally putting the medal around my neck, and he said, ‘Hey, I just sent you some money today,’” she recalled.
The new center will collaborate with companies such as AmpliPhi Biosciences and Adaptive Phage Therapeutics to treat future patients. Some of them will have cystic fibrosis, which causes mucus in the lungs to be overly sticky, often allowing drug-resistant microbes to proliferate. Others might have long-term infections that are preventing them from getting organ transplants. Yet others will have implanted devices, which sometimes provide the nooks and crannies where bacteria can grow into a slimy film.
“The sad thing is that there is going to be no shortage of patients,” said Strathdee.