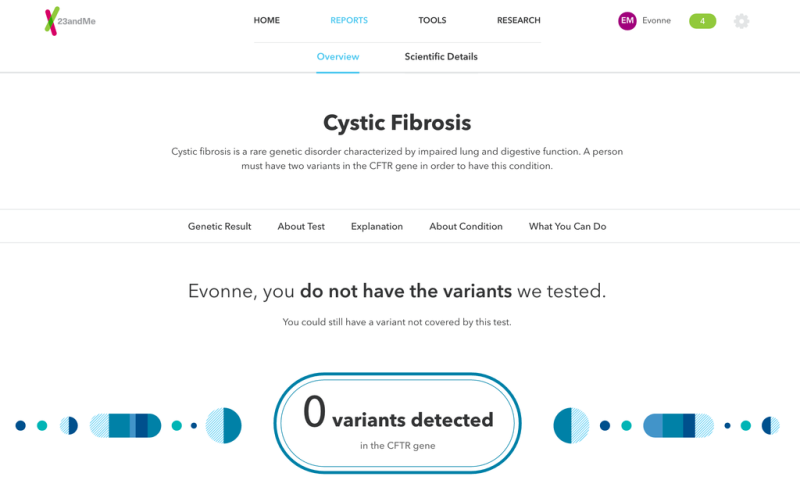
The genetic testing service 23andMe has relaunched a controversial test that, using just a few drops of spit, can tell you if you're a carrier for dozens of diseases.
It's not as extensive as its previous service, which could screen for hundreds of health risks, but the new modified test is approved by federal regulators, clearing a major hurdle that had placed the company's future in doubt.
In November of 2013, the startup was hit with a warning letter from the Food and Drug Administration, ordering the company to stop selling and marketing its personal genome service, which tested for risks associated with diseases like breast cancer and Parkinson's Disease. While the company pondered how to prove the accuracy of its product to the FDA's satisfaction, 23andMe stripped down its genetic test to only deliver raw genetic data and ancestry information. The sales of its testing kits dropped.
"We didn't understand the implications of that letter," said 23andMe's president Andy Page, in an interview earlier this week. "We needed to hire a lot of people to get us back on track."
Since then, 23andMe has worked closely with regulators to bring its full test back to market. Earlier this year, the FDA approved its carrier test for Bloom Syndrome, a rare disease associated with short stature and a higher cancer risk. At the time the FDA said it would not review other such carrier screening tests, clearing the way for the company to resume offering some health information.
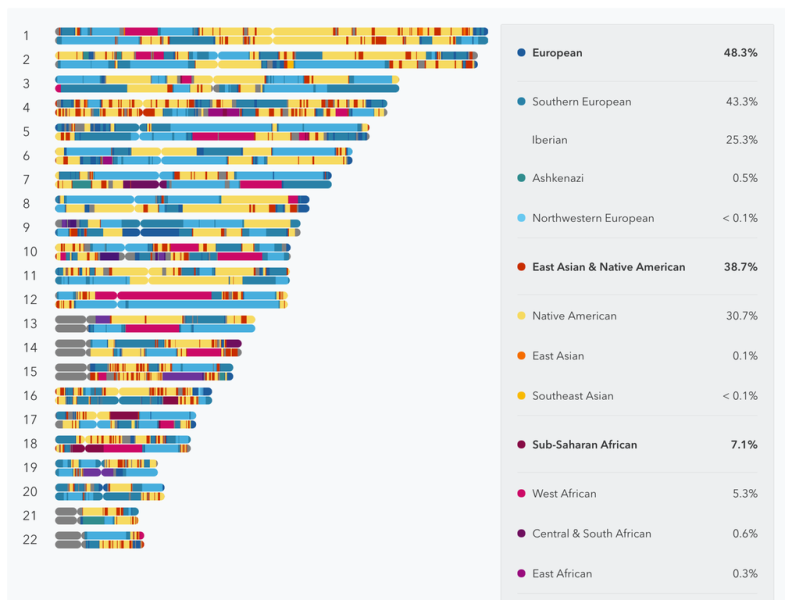
Earlier this week, the company walked me through its redesigned product, which is available for $199. 23andMe bills this test as a major improvement on its previous efforts, despite that its roster of health tests is limited. It still doesn't include carrier tests for hereditary breast and ovarian cancer, as well as tests for drug responses and adverse drug reactions. Prior to the regulatory crackdown, 23andMe included these tests and more.