"I was immediately told that they have 50 vaccines available today and people who came earlier already got it," he said. "It disappeared in just a second and they're not sure about a new shipment."
To date, the U.S. government has purchased more than 1.1 million complete doses of the vaccine, which is produced by Bavarian Nordic in Denmark. But the company said earlier this week it needed authorization from on-site FDA inspectors before it could begin shipping most of them.
An FDA spokesperson said late Wednesday that regulators had "expedited and completed an inspection of the company's plant."
"We do not expect any delay in vaccine availability due to this process," she said in an emailed statement.
Bavarian Nordic has already shipped to the U.S. 300,000 doses that were made at a third-party facility that had previously been inspected by the FDA.
The U.S. government has shipped 132,000 doses of the vaccine to health departments, including more than 21,000 to New York City and nearly 27,000 to California. Federal officials are distributing the vaccines based on each area's case numbers and the portion of the population that is at higher risk from the virus.
But those limited vaccine supplies aren’t keeping pace with the growing number of people seeking appointments, fueling growing anxiety about the virus, according to health officials in areas of the country where demand has spiked.
"The demand has been very, very high — overwhelming, at this point," said Dr. Mary Foote, of the New York City Health Department. "We just want to make clear: If we have the vaccine, we can administer it. The critical issue here right now is supply."
Earlier this week, the city's website for scheduling vaccination appointments crashed due to huge online traffic.
White House officials have promised more supplies, chiefly from the Bavarian Nordic stockpile. The U.S. recently ordered 2.5 million more doses for delivery later this year or early next year. And the company says it has enough bulk ingredient to make roughly 15 million more doses for the U.S.
Monkeypox is "rarely fatal," according to the CDC, which says that "over 99% of people who get this form of the disease are likely to survive." Most patients experience some fever, body aches, chills and fatigue. People with more serious cases may develop a rash and lesions on the face and hands that can spread to other parts of the body.
U.S. officials are now partnering with several large commercial testing laboratories and say they expect to be able to process 70,000 tests per week by the end of the month.
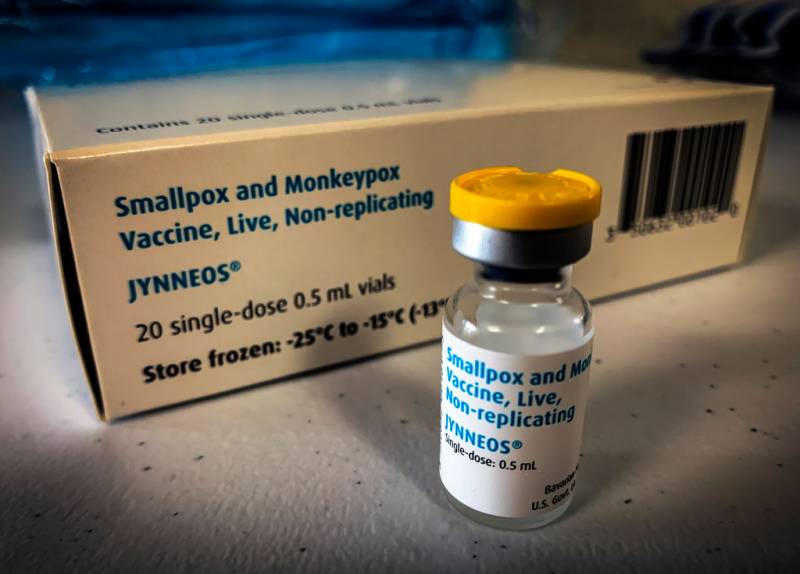
Bavarian Nordic's Jynneos, one of two monkeypox vaccines approved for use in the U.S., is considered to have fewer potential side effects and has been the primary weapon against the outbreak.
The CDC recommends the vaccine for people who have already been exposed to the virus and their presumed contacts. That includes men who have recently had sex with other men they've recently met, in cities where monkeypox cases have been identified.
Health experts say they eventually hope to see a broader vaccination campaign to preemptively protect people at high risk.
"There is not enough vaccine to vaccinate the entire population, but definitely categorizing higher-risk groups and prioritizing them will be essential to use our resources wisely," said Dr. Lilian Abbo, an infectious diseases expert at the University of Miami.
This story includes reporting from Matthew Perrone of The Associated Press, and KQED's Carlos Cabrera-Lomelí, Vanessa Rancaño and Spencer Whitney.