When infected with a virus or other pathogen, our immune system makes proteins called antibodies to fight off the infection. Now, scientists and health officials say newly authorized blood tests for COVID-19 antibodies may be key to tracking the spread of the virus and figuring out who could return to work.
COVID-19 Antibody Testing Ramps Up in California
Thanks for signing up for the newsletter.
A growing number of academic and private labs in California have begun running serological, or blood, tests for COVID-19 antibodies, including Stanford University as well as USC in conjunction with the Los Angeles County Department of Public Health.
Unlike nasal swab tests that detect who currently has the virus, antibody tests can capture those who already had it but were asymptomatic or never tested.
“That will give a lot more information about penetrance in the community,” said Spenser Smith, lab director at ARCpoint Labs of Monterey. The small private lab began running the skin-prick test last week on frontline health workers and other first responders. Tests are available by appointment or referrals from health care providers.
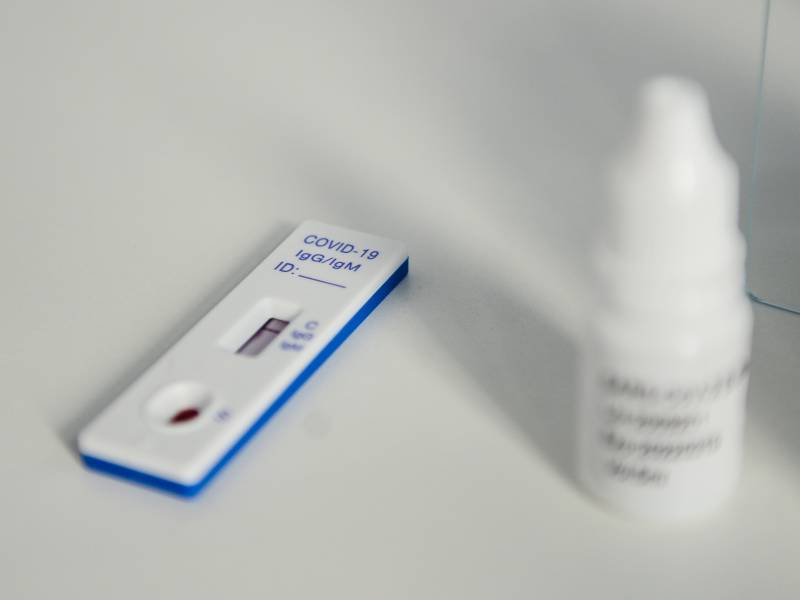
“You just have to poke on the finger, a couple of drops of blood and it kind of looks like a pregnancy test,” he said. “You’ll see a little line there denoting it was positive for those antibodies.”
Smith says the test looks for two distinct COVID-19 antibodies, one the body produces to fight the virus right away and another that could be important for lasting immunity.
“We’re hopeful that once you’re exposed, you’ll be immune, at least for the season,” he said.
While the science behind antibody tests is well-established, researchers are still trying to determine if COVID-19 antibodies prevent reinfection and for how long.
The FDA recently authorized the emergency use of COVID-19 serological testing in the U.S., but more data is needed to determine the reliability of some of the tests that are out there.
Dr. Charity Dean, assistant director of the California Department of Public Health and co-chair of the state’s testing task force, says once there’s more data, the state could begin using antibody tests to help determine who can stop sheltering at home.
“One of the benefits of doing the serology test in California is if these tests do, in fact, reflect immunity by someone who has been previously infected, it will help give California data that can inform our strategy,” Dean said.
“Our hope is that the test to detect the antibody will actually be detecting immunity and be able to be scaled up…so that people who have been infected can go back to providing the critical services that they provide without the risk of becoming reinfected.”
Smith’s lab has focused on frontline workers who have a high risk of exposure. He says ARCpoint is running about 150 tests a day via drive-through, and that some people have tested positive for COVID-19 antibodies. The lab is sharing that data with the Monterey County Health Department.
Researchers at Stanford University recently launched multiple projects to study and deploy serology tests for COVID-19 antibodies.
Dr. James Zehnder, director of clinical pathology at Stanford, says the university validated an antibody test through a rigorous study in its clinical lab over the course of two weeks. This means they tested for antibodies in people with known coronavirus infections, as well as testing a control group who did not have the virus.
The university says first priority for antibody testing is given to health care workers, but testing is open to the entire Stanford community — including students, faculty and staff. Stanford can currently run about 500 tests per day, but plans to increase capacity soon.
Results from the lab test, which requires a blood draw, are ready within two to three days. Zehnder says while the wait is longer than point-of-care skin-prick tests, the results are potentially more accurate.
“There’s been a proliferation of these point-of-care tests, and I think one concern that we have is that they need to be compared to sort of gold standard testing in clinical laboratories,” he said.
Zehnder says antibody tests will help researchers determine the rate of COVID-19 infections in the region, accounting for the potential number of asymptomatic cases.
“There’s two goals, really,” he said. “To ensure the safety of the workforce, and also to get a better idea in the community what the status of this disease is and how people are responding to it.
Since it can take two to three weeks for an infected person’s immune response to kick in and for antibodies to be detected, he says it can be done in tandem with nasal swab testing.
“Having both kinds of data allows you to have a more accurate picture of what’s going in an individual person at a moment in time,” Zehnder said.
Serological testing may be key to developing vaccines and plasma treatments for the disease using antibody-rich blood. It could also help scientists determine if the population has lasting immunity. But Zehnder says we’re not there yet.
“These are research questions that everyone in the world wants to know the answer to. So we’re in the data collection phase now. And there’s researchers at Stanford and many institutions that are working together on this.”
