Delaney Van Riper’s genes contain an error. Were we to look at her genetic code in a Microsoft Word document, it would be really hard to find the written misspelling. On a single page of that genetic code, just one letter is wrong, but that letter translates into a degenerative nerve condition. The nerves that run to Delaney’s hands and feet are damaged, affecting how she walks and moves and writes and lives. Now Delaney and her parents are among the first to grapple directly with the question: What does it mean to be able to spell check your genetic code?
From the moment Delaney was born, her father AJ says, she was a force of energy and motion. He called her a “bowling ball.” She flew around the house, running, pushing, dancing and laughing. That’s why it almost went unnoticed that as a young child, Delaney walked mostly on her tiptoes.
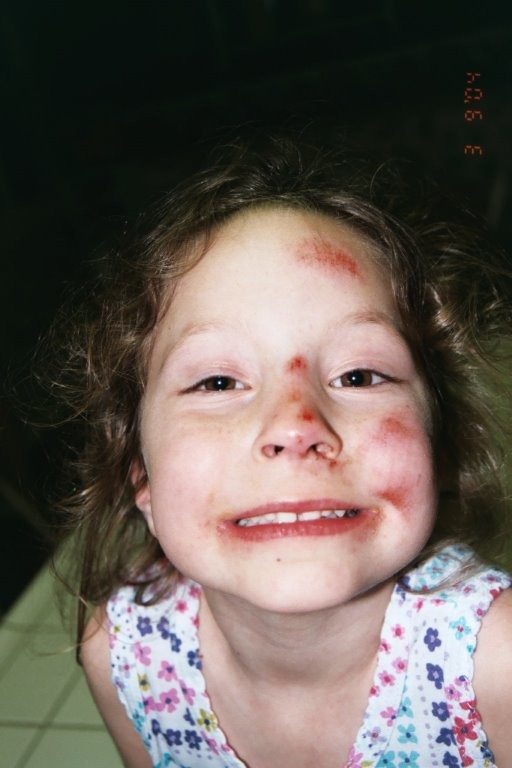
“One day, the parent side of me switched into the genetic counselor side of me. I was like, ‘Do I have a daughter who toe-walks?’ ” AJ remembers.
AJ Van Riper is a genetic counselor, a health care professional with specialized training in medical genetics, who acts as a sort of therapist and adviser for people with genetic disorders. Which is how he knew that when children walk exclusively on their tiptoes, it can be a sign of an underlying genetic disorder.
After extensive testing, Delaney, then 7 years old, was diagnosed with CMT, which stands for Charcot-Marie-Tooth and is characterized by progressive muscle degeneration and weakness. It’s a genetic disorder that impacts the nerve signals going to Delaney’s arms and legs. Delaney has muscle atrophy and tightened ligaments, which makes her hands and feet look slightly curved and thin, and makes her prone to tripping and falling.
CMT is one of the most common inherited neurological disorders, and over 1,000 genetic misspellings can cause the disorder. But only about 12 people in the world have the exact misspelling that Delaney has, a mutation called “P182L.”
When Delaney was a kid, she wore leg braces. She says she felt bionic.
“When you’re young, you want to be cool, different from everybody else,” Delaney says. “I was different from everybody else, but eventually it became not in a good way.”
As she grew, Delaney started to trip and fall more often. When she hit puberty, her hands started to weaken. She became more aware of these snagging limitations on her body. She could no longer hold a pencil. The teenagers around her noticed. A parade of negative thoughts took over, she says, led by “Why me?”
Neither of Delaney’s parents has CMT. Rather than being hereditary, as is common, her disorder was a unique mutation in her DNA. Delaney recognized during those difficult teenage years that she had nobody to blame for her disorder, but that just forced her anger inward. She isolated herself and became depressed, even veered into self-harm.

Through personal writing, Delaney began to come to terms with the genetic hand she was dealt. At age 16, she wrote about her depression and her disorder in a magazine called CMTeen:
I’ve not yet won my war... I am repairing myself. I can feel when the bad days are coming and I prepare. I survive my struggles because I have done so for 10 years. Each day for 10 years, I woke up and got out of bed. Why not try for 10 more? I know I can do this.
Delaney found solace in the fact that her disorder is, as she calls it, “a fluke,” that neither she nor her parents had any fault in the matter. She had to let go of her desire to be anything but herself. Delaney tells me now, “I had to accept that this is going to be my life for as long as I know.”
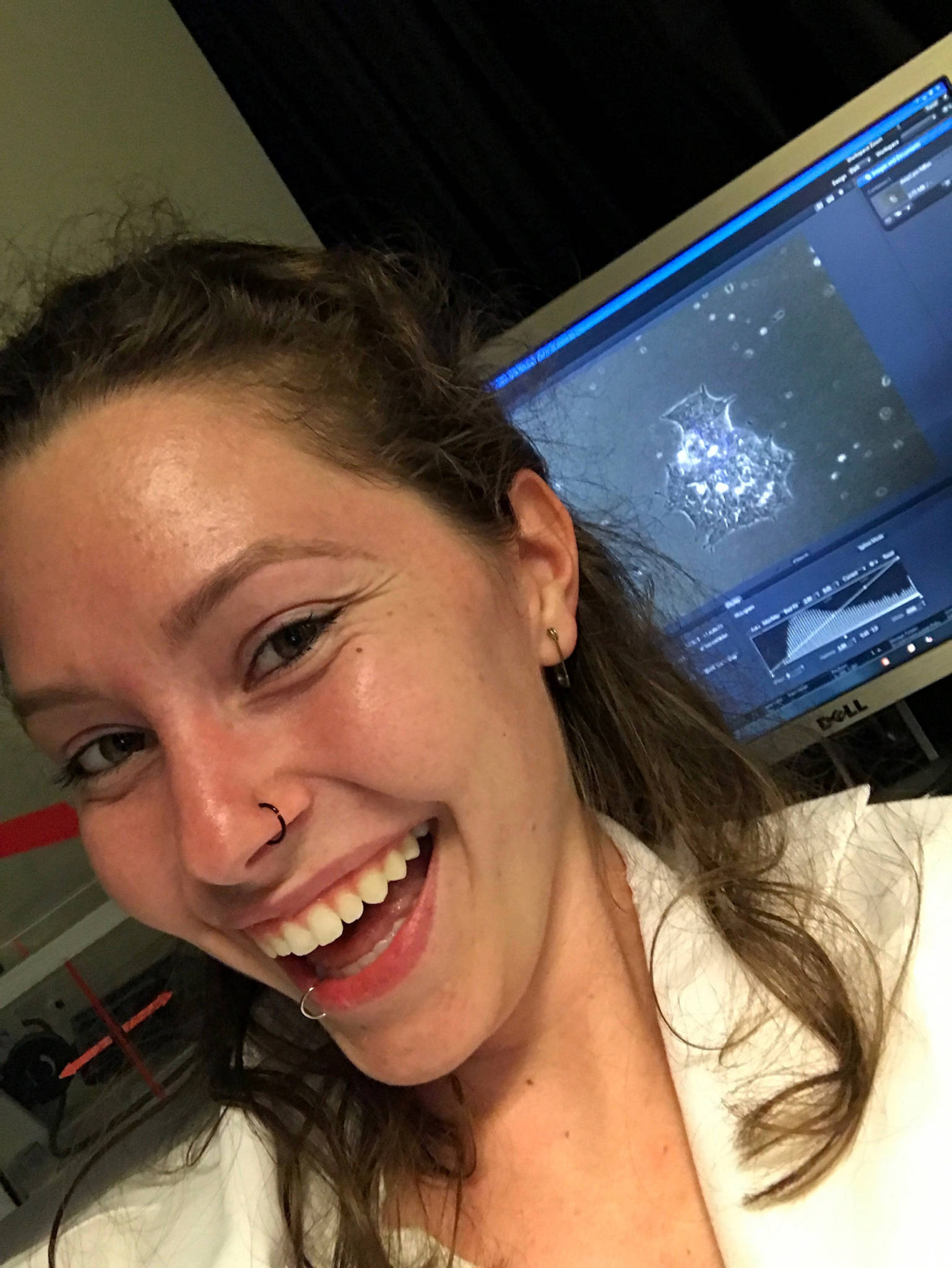
Delaney is now 20 years old, a sophomore at UC Santa Cruz, studying literature. When Delaney was 17, she was surprised by an email from a Bay Area scientist, Dr. Bruce Conklin. He was looking to find patients for a study, people with CMT, especially the incredibly rare kind of CMT that Delaney has. Conklin hoped to one day remove the genetic mutation from patients’ bodies.
“It was presented as a possible research study that, if everything goes well, it could be a cure, but also to be realistic at the same time,” Delaney says. Her feelings about herself and her CMT shifted again. “The worst that could happen is I just kind of stay the same.”
Conklin is a professor at UCSF and senior investigator at San Francisco’s Gladstone Institutes, which is partnered with CRISPR pioneer Jennifer Doudna and The Innovative Genomics Institute. Delaney is now one of a handful of patients who are part of Conklin’s study.

Conklin calls his practice “gene surgery” both because it’s an anatomical removal of something, just on a genome scale, and because he considers surgery to be a process toward eradicating a disease.
“When people did the first heart transplant, they didn't think about how to monetize the 10th heart transplant,” Conklin says. “They thought about how to make the next patient live a little bit longer, the next patient live a bit longer.”
He says that his practice is like the beginning stages of transplant surgery, working out the steps in what might someday become common practice.
Not all gene surgery is created equal, in terms of how scientists measure its potential risks. Conklin’s lab focuses its efforts on somatic gene editing, as opposed to germline editing. Germline editing was controversially employed in 2018 by Chinese scientist He Jiankui, who edited the genome in human embryos and implanted them in a woman who gave birth to twins.
Germline editing involves editing a human embryo, which affects every single cell in the developing embryo’s body, and these edits will be passed to future generations. Somatic editing, on the other hand, affects only the type of cell that is edited, only in one person’s body, and the changes cannot be inherited by future generations.
In March, a committee of the World Health Organization assembled after the controversy to review the state of human genome editing projects.
“The committee agrees that it is irresponsible at this time for anyone to proceed with clinical applications of human germline genome editing,” a committee co-chairwoman, Dr. Margaret Hamburg, said in a press briefing.
The committee also resolved that all human gene-editing projects should be listed in a mandatory global registry.
The field is progressing more quickly than most scientists could have predicted, Conklin says.
“[Gene editing] is not easy to do safely and ethically, but it's remarkably easy to do," he says. “The thing that really shocked people about the unethical experiment in China was that the added costs, in addition to the in vitro fertilization, which already was going on, was only about $500.”
There are roughly 6,000 disorders that involve a mutation on just one gene. Conklin is starting with rare diseases like CMT. Biotech companies are focused on more common disorders whose gene editing therapies can be monetized more easily.
“It leaves the vast majority, probably 98% of the diseases essentially to us to work on. I think that that's fine,” Conklin says. “We just have to seize the moment.”
But just because a disease is rare doesn’t mean it’s not worth editing. Actually, Conklin sees the study of rare disorders like CMT to be a critical part of moving gene editing forward. He’s starting with CMT in part because editing this particular genetic disorder is straightforward.
Our chromosomes carry two copies of every gene, and only one copy of Delaney's gene has a typo. Editing that typo just involves cutting away part of the genome, not replacing it, which is a much more complicated process.
“With Delaney, we know exactly the genome to edit,” Dr. Conklin says. “We know that we can edit it. We know roughly how to deliver it.”
There’s an ethical conflict that Delaney believes is worth exploring now, one that is inherent in any gene-editing process. Who decides what needs to be edited, and what psychological harm might it do to frame genetic disorders as mistakes to be fixed?
Indeed, the entire framework of gene editing is infused with the sense that editing is "fixing." It’s finding an error in the genetic code and correcting that error. Fixing is part of her family’s hope for an “easier” life for her. Both of her parents say that they wish they could go back in time and remove the disorder from her genes.
“If I had the power to go back on a time machine and touch your shoulder and lift CMT out of you, I would do it in a heartbeat,” AJ tells Delaney during an interview in their Sacramento home.
Delaney responds that CMT is a deeply important part of who she is, including the depression and self-harm it caused. She attributes her strength to the error in her genes.
“You're not really fixing us,” Delaney says. “I know it's the easiest way for people to understand, but there's nothing really to fix.”
Still, as proud and secure as Delaney has become with her disorder, she now wants to edit it out.
“I would definitely edit my genes,” she says. “I've learned my lessons, I think. I know who I am as a person, and I just don't want my life to be as difficult as it has to be.”
It’s complicated, Delaney realizes. But most important to her is that gene editing be framed as a choice for people with disabilities, rather than assuming everyone would want their genes edited.
“It's not all negative. It's not all sad. And just because I have it doesn't mean I can be less happy in life. It just means my life is more difficult,” Delaney tells me. Then she adds, “But difficulty doesn't always equal sadness.”
